Marketed By
novartis
Pack of
14 Tablet
Salt Composition
Eltrombopag Olamine
Storage
Keep in cold place
₹13900₹15656
11.22% Off
Inclusive of all taxes
1
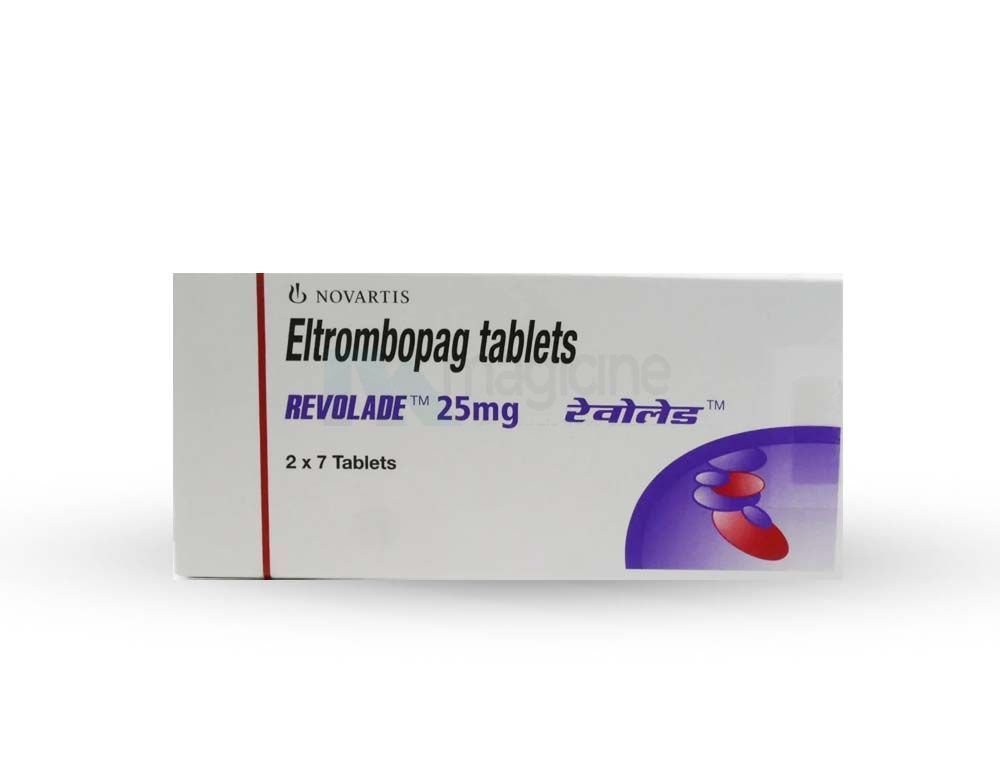
Revolade 25mg Tablet
Delivering To: —
All Substitutes
Overview
Revolade 25mg Tablet is used to treat low platelet count due to chronic immune (idiopathic) thrombocytopenic purpura (ITP) or chronic hepatitis C virus (HCV) infection. It is also used to treat severe aplastic anemia. Revolade 25mg Tablet is a prescription-based anticoagulant drug used to increase low platelet count in the blood that is caused due to primary immune thrombocytopenia (ITP) or hepatitis C (HCV) infection. It is also given to patients to cure severe aplastic anemia.
Indication
Hepatitis C, Hepatitis Medicines, Low Red Platelet Count
Uses
1. Chronic idiopathic thrombocytopenia
2. Treatment of Severe Aplastic Anemia
3. Low platelet count due to ITP or HCV infection
Side Effects
Like other anticancer medicines, Revolade 25mg Tablet also has some common to severe side effects. These are undesired symptoms observed in the body after taking this injection. Usually, they disappear with time as the body adjusts to the medicine. These adverse effects don't need special medical attention, but if you observe them continually, talk to your medical expert. Some of them are listed below: Common side effects:
Diarrhea
Back pain
Appetite loss
Hair loss
headache and dizziness
Throat pain
Nausea & vomiting, muscle pain
Abdominal pain
Cold and cough
Upper respiratory tract infection
Severe side effects:
Urinary Tract Infection
Increased liver enzymes
How To Use
Revolade 25mg Tablet is an oral anticancer medication that requires a prescription. It is recommended that you speak with your healthcare provider before using this tablet. As directed by your physician, take this medication for the prescribed amount of time. Eat it all at once. Avoid chewing, crushing, or breaking it. The Revolade 25mg Tablet should be taken without food.
How It Works
Revolade 25mg Tablet belongs to the class of thrombopoietin (TPO) receptor agonists, consisting of the active ingredient Eltrombopag. It stimulates the bone marrow cells, called megakaryocytes. It is responsible for producing platelets in the blood. Further, Revolade Tablet prevents the loss of blood and bruising. Revolade 25mg Tablet is an anticoagulant drug belonging to the class of thrombopoietin (TPO) receptor agonists. The doctor prescribes this tablet to increase the blood platelet count caused due to chronic immune (idiopathic) thrombocytopenic purpura (ITP) or chronic hepatitis C virus (HCV) infection. ITP is an autoimmune disorder in which our immune system starts damaging our platelets. Platelets are blood cells that help in blood clotting and protecting vessels. When there is a low count of platelets in the blood, it can cause bleeding. Revolade 25mg Tablet is also used to treat severe aplastic anemia. It is a severe blood disorder that occurs when the bone marrow fails to produce enough new blood cells. It is advised not to take this tablet if you have liver disease, as Revolade 25mg Tablet can cause hepatotoxicity, increased liver enzymes, and bilirubin levels. It may worsen liver function. Your doctor must go for a weekly eye checkup for bleeding near the retina. Inform your doctor if you are taking any prescribed and non-prescribed medications. These medicines can interact with Revolade 25mg Tablet and may result in some undesirable effects. This tablet is not recommended for people under age 18. Revolade 25mg Tablet should not be given to children under age one to treat ITP.
Safety Advice

Alcohol
consult your doctor
The interaction of alcohol with this drug is still unknown. It is advisable not to consume alcohol while taking Revolade 25mg Tablet. Kindly consult your doctor for further information. The Revolade 25mg Tablet with alcohol may interact; however, this is uncertain. If you consume alcohol, let your doctor know.

Pregnancy
consult your doctor
Revolade 25mg Tablet may be considered unsafe during pregnancy. Please consult your doctor in such a condition. Due to the possibility of reproductive damage, Revolade 25mg Tablet is not advised for use by expectant mothers. If you are pregnant, suspect, or want to become pregnant while undergoing treatment, you must let your doctor know.

Breast Feeding
consult your doctor
Revolade 25mg Tablet is probably not safe if you're breastfeeding. Kindly consult your doctor. The Revolade 25mg Tablet's ability to transfer into breast milk is unknown. Inform your doctor if you are breastfeeding or intend to breastfeed.

Driving
unsafe
It is advisable not to drive or operate heavy machinery as you may feel dizzy after taking Revolade 25mg Tablet. After taking the Revolade 25mg Tablet, it is dangerous to drive or operate heavy equipment. It can make you feel lightheaded and make it harder to focus.

Kidney
safe if prescribed
Share your medical history with your healthcare expert in case of kidney disease before taking Revolade 25mg Tablet.

Liver
safe if prescribed
Inform your doctor before starting Revolade 25mg Tablet if you have liver disease to avoid any serious side effects. Patients with serious liver conditions should use Revolade 25mg Tablets with caution. Severe liver failure requires dose adjustment. Before starting the treatment, let your doctor know if you have liver problems.
Missed Doses
It is advised not to miss any scheduled dosage of Revolade 25mg Tablet. In case you miss a dose, consult your doctor immediately. Don't overdose to compensate for the missed one. Contact your doctor or any healthcare provider and seek medical attention immediately in such cases.
Quick Tips
Monitor your blood platelet count regularly. Taking Revolade 25mg Tablet can drop in the count.
It may take multiple weeks or several months to observe the benefits, so do not stop taking it until your doctor asks you to do so.
It is not recommended to overdose if you miss the prescribed one. Take a dose if you miss it in less than 12 hours. If 12 hours have passed, take the next day's dose.
It is advised to take Revolade 25mg Tablet at least 2 hours before or 4 hours after eating dairy products and calcium-fortified juices.
Consult with your healthcare professional before taking Revolade 25mg Tablet if you are planning to be pregnant, planning to be pregnant or breastfeeding. It is recommended to use effective birth control methods to avoid pregnancy.
This drug is not recommended for people with hypersensitivity and allergy.
FAQs
Revolade 25mg Tablet is an anticoagulant drug used to increase low platelet count in the blood, usually caused by primary immune thrombocytopenia (ITP) or hepatitis C (HCV) infection. It is also given to patients to cure severe aplastic anemia.
Yes, Revolade 25mg Tablet increases the number of platelets by stimulating megakaryocytes, the bone marrow cells responsible for producing platelets.
No, Revolade 25mg Tablet is not a blood thinner. It can't liquefy the consistency of the blood. This tablet raises the number of platelets in the blood. That makes blood thicker and prevents blood loss.
It is advised not to take dairy products and calcium-fortified juices at least 2 hours before or 4 hours after taking Revolade 25mg Tablet.
Blood platelet count and liver enzyme must be monitored regularly during treatment with Revolade 25mg Tablet. Your doctor must go for weekly cataracts and eye checkups for bleeding near the retina.
Revolade 25mg tablets should not be used by children younger than one year old or by those with immune system disorders such as primary thrombocytopenia (ITP). Additionally, it is not advised for those who have hepatitis C and severe aplastic anemia.
Yes, you should take the 25 mg Revolade capsules without food. Because this drug absorption can be blocked by antacids, dairy products, calcium-enriched diets, fortified drinks, and mineral and vitamin supplements (iron, calcium, magnesium, aluminum, selenium, and zinc). Therefore, please take them four hours after taking the drug or at least two hours before.
Yes, you will be closely watched when taking the 25 mg Revolade tablet. Your complete blood count, heart rate, eye test, platelets, blood clotting factors, and white blood cells will all be routinely evaluated by your healthcare provider. Depending on how you react to the drug, a 25 mg substitute for Revolade can be taken into consideration.
Trusted Medicines, Delivered Worldwide
At our pharmacy, your health is our top priority. All of our medicines are safe, effective, and approved by leading health authorities. We partner exclusively with reliable and reputable manufacturers, ensuring the highest quality standards in every product we deliver.
Enjoy the convenience of fast home delivery, secure packaging, and affordable prices—plus special discounts on bulk orders. We’re proud to offer international shipping to a wide range of countries across Asia, Europe, Africa, the Americas, and beyond, making trusted healthcare accessible no matter where you are.
Whether you're a patient or a healthcare provider, you can order with confidence and compassion. Our goal is to ensure that quality healthcare reaches everyone, everywhere.
We currently ship to:
Barbados, Haiti, Trinidad and Tobago, Mexico, Bahamas, Ecuador, Peru, Chile, Brazil, Colombia, Philippines, U.K., USA, Kuwait, Saudi Arabia, UAE, Oman, Qatar, Japan, Ireland, Russia, China, France, Australia, Lithuania, Nigeria, Romania, Algeria, Canada, Singapore, Thailand, Hong Kong, Bahrain, Jordan, Indonesia, Hungary, Moldova, Bulgaria, Azerbaijan, Congo, Ethiopia, Lebanon, Morocco, Mozambique, South Korea, Sri Lanka, Belarus, Mongolia, Malaysia, Vietnam, Tunisia, Zambia, and Israel.
Experience the quality everyone’s talking about – order today!
Reference
Bussel, J. B., Cheng, G., Saleh, M. N., Psaila, B., Kovaleva, L., Cheng, G., Kuter, D. J. (2007). Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. New England Journal of Medicine, 357(22), 2237–2247.
Eltrombopag Olamine (Revolade). (n.d.). Results – ENABLE 1 and ENABLE 2 trials. In NCBI Bookshelf. Retrieved from NCBI Bookshelf
European Medicines Agency (EMA). (n.d.). Revolade: usage, mechanism, and benefits in ITP and related conditions. Retrieved from the EMA website
Kuter, D. J., Bussel, J. B., Lyons, R. M., et al. (2008). Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: A randomized, double‑blind, placebo‑controlled trial. Blood, 111(11), 5327–5333.
Medicines Information Source (MIMS HK). (n.d.). Eltrombopag in hepatic impairment: initiating at 25 mg once daily. Retrieved from MIMS Hong Kong
Park, S., Brümmendorf, T. H., Fenaux, P., et al. (2017). Eltrombopag versus placebo for low‑risk myelodysplastic syndromes with thrombocytopenia (EQoL‑MDS): Phase 1 results of a single‑blind, randomized, controlled, phase 2 superiority trial. The Lancet Haematology, 4(1), e22–e31.
Peffault de Latour, R., Bacigalupo, A., Socié, G., et al. (2022). Eltrombopag added to immunosuppression in severe aplastic anemia. New England Journal of Medicine, 386(15), 1392–1402
Rahman, A., Ahmed, T., et al. (2020). Investigation of the efficacy and safety of eltrombopag to correct thrombocytopenia in moderate to severe dengue patients: A randomized, controlled clinical trial. eClinicalMedicine, 25, 100457.
SmPC (RxReasoner). (n.d.). Eltrombopag (Revolade): dosage recommendations, including 25 mg initiation for specific populations. Retrieved from RxReasoner
Terazawa, S., et al. (2012). Efficacy and safety of eltrombopag in Japanese patients with chronic liver disease and thrombocytopenia: A randomized, open‑label, phase II study. Journal of Hepatology, 56(6), 1253–1261.
Related Products
MARKETER DETAILS
novartis
DISCLAIMER
The contents of this website are for informative purposes only. They are not deliberated to be an alternative to any professional medical prescription and treatment. Seek the advice of a qualified health provider for questions regarding the medical condition. Do not ignore any professional medical advice because of something you have read on this website. This website offers links to other websites, thereby enabling you to go to the other website directly. Therefore, Magicine Pharma isn't responsible for the content of the links in the website or links in the linked websites. The links are provided to assist the visitors and are not approved by any professional health provider.